Press Release
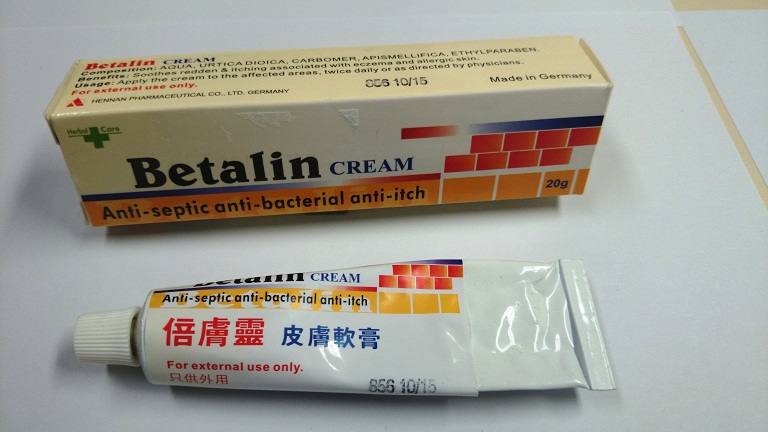
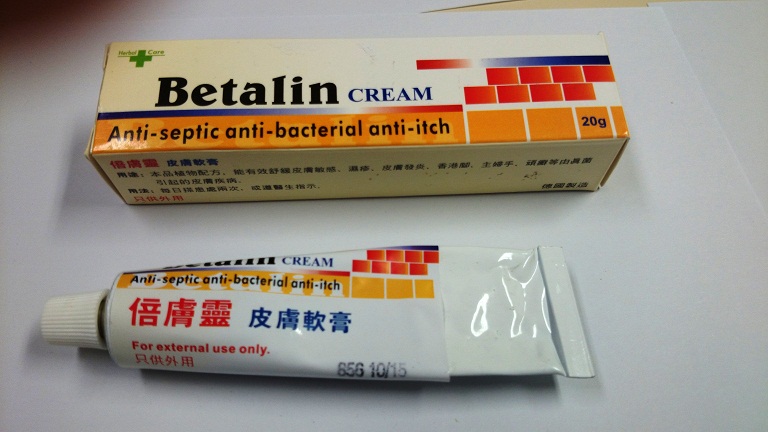
Public urged not to buy or use product labelled Betalin Cream
13 February 2014
The Department of Health (DH) today (February 13) urged the public not to buy or use a product called "Betalin Cream" as it was found to contain undeclared and controlled ingredients.
Acting on a public complaint, a pharmacy located in North Point was found to be offering the product for sale. A sample of Betalin Cream was purchased for analysis and the Government Laboratory's results showed that the sample contains tolnaftate (an antifungal drug) and a Part I poison betamethasone (a steroid), which is a prescription drug. The product is not a registered pharmaceutical product in Hong Kong. The pharmacy was raided today in a joint operation by the DH and the Police. During the operation, a 38-year-old man was arrested for suspected illegal sale of an unregistered pharmaceutical product and selling a prescription drug without prescription.
The investigation is continuing.
Topical betamethasone is used for the treatment of inflammatory skin disorders and should only be used under the advice of a doctor. The product can only be sold at pharmacies under the supervision of a registered pharmacist upon a doctor's prescription. Inappropriate use of steroids may cause serious side effects such as Cushing's Syndrome, with symptoms including moon face and muscle atrophy. Tolnaftate is commonly used for fungal infection of the skin. It can cause side effects such as irritation and allergic reaction.
According to the Pharmacy and Poisons Ordinance (Cap 138), all pharmaceutical products must be registered with the Pharmacy and Poisons Board of Hong Kong before they can be sold legally in the marketplace. Illegal sale and possession of unregistered pharmaceutical products or illegal sale of prescription drug are criminal offences. The maximum penalty for each offence is a fine of $100,000 and two years' imprisonment.
A DH spokesperson strongly urged members of the public not to buy or use products of doubtful composition or unregistered pharmaceutical products as their safety, quality and efficacy may not be guaranteed. All registered pharmaceutical products should carry a Hong Kong registration number on the package in the format of "HK-XXXXX".
Members of the public who have purchased the above product should consult healthcare professionals for advice. They may submit the product to the DH's Drug Office at Room 1856, Wu Chung House, 213 Queen's Road East, Wan Chai, during office hours for disposal.